

Solutions in Water Environment
for People and Planet



Electroplating wastewater is the process of electroplating the surface of metals used in various industries to produce metal parts with specialized features required by different industries.

The steel industry is categorized into various production sectors, such as rolled steel, special steel, cold-rolled and galvanized steel, stainless steel, steel pipe, hot rolling, wire rod, and alloy steel, where electroplating is a technology for secondary processing.
The purpose of electroplating is to ‘build up a film" of chromium, nickel, copper, gold, and other materials that can fulfill the function of the intended application.
The functions that can be achieved from electroplating include rust resistance, strength, wear resistance, and electroconductivity, and constant, pure, and ultrapure water is also used in semiconductor applications.
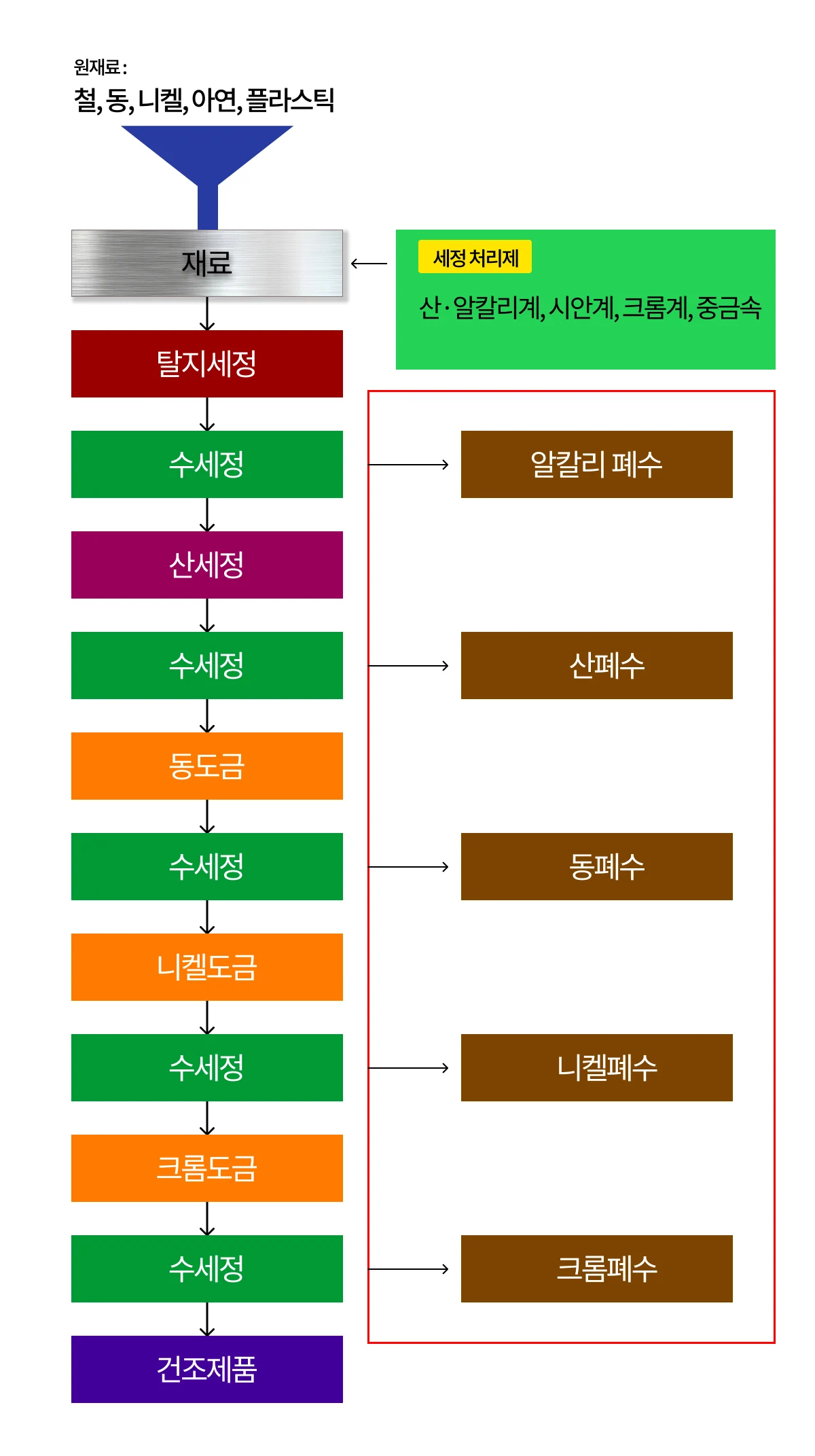
As in the previous metal surface treatment industry, wastewater is generated, and wastewater containing heavy metals such as acid-alkali / cyan / chromium is the main substance due to substances such as chromium and nickel used to form surface films.
The main processes of electroplating wastewater are degreasing and water washing. These two processes complete the processed products, and acid-alkali wastewater and heavy metal wastewater depending on the cleaning agent are generated when the cleaning agent is added.
2NaCNO + 5NaOCL + H₂O -> 2CO₂ + N₂ + 2NaOH + 5NaCl
4H₂CrO₄ + 6NaHSO₃ + 3H₂SO₄ -> 2Cr₂(SO₄)₃ + 3Na₂SO₄ + 10H₂O
Cyanide electroplating wastewater has the same structure as above. The above treatment process diagram is an alkaline chlorine method, which adds acid to the second oxidation step to achieve a pH of 7~8. The role of the reducing bath is to remove iron and nickel, which cannot be decomposed by alkaline decomposition, so chlorine can be used to treat nickel and iron cyanide.